

Download the alternative format
(PDF format, 734 KB, 42 pages) Organization: Health Canada Published: March 2020
GUI-0016: Guidance on Medical Device Establishment Licensing (MDEL) Author: Health Canada Date issued: April 1, 2020 Date implemented: April 1, 2020 Replaces: Guidance on Medical Device Establishment Licensing and Medical Device Establishment Licence Fees (GUI-0016) version 7 (April 1, 2013) Health Canada is the federal department responsible for helping the people of Canada maintain and improve their health. We assess the safety of drugs and many consumer products, help improve the safety of food, and provide information to Canadians to help them make healthy decisions. We provide health services to First Nations people and to Inuit communities. We work with the provinces to ensure our health care system serves the needs of Canadians. Également disponible en français sous le titre : Document d’orientation concernant l’octroi d’une licence d’établissement d’instruments médicaux (GUI-0016) For more information, please contact:
Health Canada
Address Locator 0900C2, Ottawa, ON K1A 0K9
Tel.: 613-957-2991
Toll free: 1-866-225-0709
Fax: 613-941-5366
TTY: 1-800-465-7735
Email: publications@hc-sc.gc.ca This publication can be made available in alternative formats upon request. © Her Majesty the Queen in Right of Canada, as represented by the Minister of Health, 2020 Publication date: April 1, 2020 This publication may be reproduced for personal or internal use only without permission provided the source is fully acknowledged. Cat.: H14-334/2019E-PDF
ISBN: 978-0-660-32404-3
Pub.: 190319
Disclaimer This document does not constitute part of the Food and Drugs Act or its regulations, and in the event of any inconsistency or conflict between the Act or regulations and this document, the Act or the regulations take precedence. This document is an administrative document that is intended to facilitate compliance by the regulated party with the Act, the regulations, and the applicable administrative policies.
The following table shows the three types of icons used in this document, and the way they are intended to be used.
Important: Key or cautionary information for people to know. Information: Supplementary information like quotes and legal references. Tip: Things for people to do or understand.This guidance document is intended for any person in the medical device industry and it provides guidance on regulatory requirements in relation to Medical Device Establishment Licences (MDEL), including when and how to apply for an MDEL, and how to maintain an MDEL once issued.
This guidance document does not cover importing medical devices for personal use or for use on animals.
This guidance document explains:
This guidance document covers licensing requirements for medical device establishment licences (MDEL), including who requires an MDEL.
This guidance document describes how to:
The scope of this guidance document does not cover:
The term ‘medical device’ covers a wide range of products used in the treatment, mitigation, diagnosis or prevention of a disease or abnormal physical state. See Appendix A - Glossary for regulatory definitions of a ‘device’ and ‘medical devices’.
Medical devices are categorized into four classes (I, II, III or IV) based on the level of potential risk related to their use. Class I medical devices present the lowest potential risk (for example, wheelchairs), while Class IV medical devices present the highest potential risk (for example, pacemakers).
Medical devices include a range of health products, including:
A combination product is a therapeutic product that combines a device component with any other therapeutic product components, which by themselves would be classified as a singular product.
In general, an establishment licence requirement for a combination product is associated with the classification of the product. For example:
For more information on combination products, see Policy on Drug/Medical Device Combination Products – Decisions or contact us before you submit your application (see Appendix C - contact information).
A medical device that is manufactured, sold or represented for delivering a drug, including medical cannabis, to a patient through smoking (i.e., the combustion of the drug and subsequent inhalation of the resulting smoke) is considered to be a Class II medical device as per the Notice: Classification of Medical Devces used to Deliver Drugs by Smoking posted on Health Canada’s website.
Health Canada issues two different types of licences for medical devices:
To view active MDEL holders, see the Medical Devices Establishment Licence Listing.
For a list of all current MDL holders, see the Medical Devices Active Licences Listing (MDALL).
In general, any person who imports into, or sells a medical device for human use in Canada requires an MDEL (see exceptions). You must apply for and maintain your MDEL to ensure compliance with the Food and Drugs Act and its Medical Devices Regulations. To maintain your MDEL, you must do the following:
Leasing a medical device is captured under the definition of “sell” (see section 2 of the Food and Drugs Act). If you lease or rent a medical device, you are subject to the requirements of the Medical Devices Regulations, including the requirement to hold an MDEL under section 44.
For a complete list of definitions, see Appendix A - Glossary.
The following table provides common examples/situations for when an MDEL is required under the Food and Drugs Act and its Medical Devices Regulations. See Establishment licence exemptions and Table 2: Licence requirements and exemptions below, for examples of when an MDEL may not be required.
I am in Canada. I buy medical devices from a manufacturer and/or supplier (distributor) outside of Canada and sell them in Canada. The foreign manufacturer or distributor already has an MDEL.
I am in Canada. I buy medical devices from a manufacturer and/or supplier (distributor) outside of Canada and sell them in Canada. The foreign manufacturer or distributor may not have an MDEL.
I am in Canada. I buy medical devices from a manufacturer and/or supplier (importer or distributor) in Canada and sell them in Canada.
I am outside Canada. I sell medical devices exclusively to an MDEL holder in Canada. My name is not on the label.
No licence required
I am outside Canada. I sell medical devices exclusively to healthcare facilities or retailers in Canada. My name is not on the label.
I am outside Canada. I sell medical devices to importers as well as healthcare facilities and/or retailers in Canada. My name is not on the label.
I am in or outside Canada. I sell Class II, III or IV medical devices in Canada that only have my name on the label as the manufacturer. I do not sell Class I medical devices in Canada.
I am outside Canada. I sell Class I medical devices in Canada that only have my name on the label as the manufacturer. I do not sell Class II, III or IV medical devices in Canada.
I ship my devices directly to the Canadian retailer.
I am outside Canada. I sell Class I medical devices in Canada that only have my name on the label as the manufacturer. I do not sell Class II, III or IV medical devices in Canada.
My client (importer/distributor) has an MDEL.
No licence required
I manufacture medical devices in Canada. I sell Class I medical devices in Canada that only have my name on the label as the manufacturer. I do not sell Class II, III or IV medical devices in Canada.
My client (distributor) has an MDEL or I sell directly to the ultimate consumer.
No licence required
An MDEL provides Health Canada assurance that medical devices sold or imported into Canada meet the safety requirements set out in the Medical Devices Regulations, and that procedures are in place to protect the public should a problem with a device be identified.
It also ensures that Health Canada is made aware of:
Classification requirement
Your Medical Device Establishment Licence (MDEL) application must list the classes of medical devices for each manufacturer or supplier that you plan to import or distribute in Canada.
As an importer or a distributor of medical devices in Canada, it is your responsibility to contact the manufacturer for further information if you are uncertain of the classification of a medical device you intend to sell or import into Canada for human use.
If a medical device falls into multiple classes, the higher risk class will apply. For example, when a medical device is classified as both a Class III and Class IV, the final classification of the medical device will be Class IV.
Manufacturers are required to take reasonable measures to identify the risks inherent in a medical device and should be able to provide the classification for any of their medical devices being sold in Canada.
Links to guidance documents for medical device classification:
It is the responsibility of the applicant to determine if they require an MDEL (for example, the medical device class and licensable activities you will be conducting). Fees for the examination of an MDEL application cannot be refunded once an application has been reviewed by Health Canada.
MDEL holders must demonstrate to Health Canada that they have met the regulatory requirements and have documented procedures in place, where applicable, related to the medical devices that they import or distribute (sell).
Manufacturers of Class II, III or IV medical devices only require a Medical Device Licence (MDL) to import or distribute their own medical devices in Canada.
It is your responsibility, as a regulated party, to understand your obligations under the Food and Drugs Act and its Medical Devices Regulations and to abide by them. Failure to comply with these obligations will lead to compliance and enforcement actions in accordance with the Compliance and enforcement policy for health products (POL-0001).
Health Canada conducts inspections of MDEL licence holders to determine their compliance with the Medical Devices Regulations. Any party conducting a regulated activity can be inspected at any time. Guidance regarding the inspection process can be found here: How Health Canada inspects medical device establishments (GUI-0064).
The following person are exempt from holding a Medical Device Establishment Licence (MDEL) under the Medical Devices Regulations to import into, or sell a medical device in Canada:
See Table 2 below, for examples of MDEL/MDL requirements and exemptions based on activity type.
An establishment contracts a person to make a Class I medical device.
The contractor does not need an MDEL.
The establishment whose name is on the label is the legally recognized manufacturer and must hold an MDEL (unless otherwise exempted).
An establishment contracts a person to make a Class II, III or IV medical device and ship to an address.
The contractor does not need an MDEL.
The establishment whose name is on the label is the legally recognized manufacturer and must hold an MDL as the private label manufacturer.
An establishment manufactures Class I, II, III or IV medical devices.
The establishment must hold an MDL for the Class II, III or IV medical devices they manufacture.
They must also hold an MDEL to sell their own Class I medical devices (unless these are solely distributed through another MDEL holder).
An establishment imports medical devices into Canada to later export to other countries from Canada.
The establishment must hold an MDEL as an importer. The manufacturer must hold an MDL in respect of its Class II, III or IV medical devices prior to its importation.
An establishment sells medical devices to hospitals, other health care facilities, or healthcare professionals/first responders.
The establishment must hold an MDEL. The hospital is not the ultimate consumer.
A hospital imports medical devices for use on patients.
An MDEL is not required. Health care facilities (for example, hospitals) are exempt, but the Class I manufacturer or distributor from whom the hospital purchased the Class I medical device must hold an MDEL.
Manufacturers of a Class II, III or IV medical device must hold an MDL to import or distribute their own medical device to the hospital in Canada.
A medical supply store rents or loans medical devices to patients.
The medical supply store does not need an MDEL.
Rent and loan is considered a sale. In this scenario the sales are to the ultimate consumer, so they are considered retail sales and are exempt.
A medical supply store is renting or loaning medical devices to hospitals/healthcare professionals or first responders, including for temporary or trial use.
The medical supply store must have an MDEL.
Rent and loan is considered a sale. The hospital/professional is not the ultimate consumer.
An establishment distributes or imports used medical devices.
The establishment must hold an MDEL.
It does not matter whether the devices are new or used.
An establishment supplies a dispenser with materials that the dispenser then uses to make medical devices.
The establishment does not require an MDEL if the materials are not medical devices themselves.
For example, contact lens buttons/blanks and hearing aid circuits are not themselves medical devices.
Sections 4 to 9 of this guidance document explain how to apply for and maintain a Medical Device Establishment Licence (MDEL). It outlines the requirements for each licensable activity related to an MDEL, including applying for, cancelling, re-instating, and keeping an MDEL up-to-date and valid.
Below is a description of the five types of MDEL applications.
Required for new applicants. Also required if you are resuming the sale of medical devices after your previous licence was cancelled by Health Canada, or cancelled by you, as the licence holder.
If your MDEL was cancelled because the licence was suspended for a period of more than 12 months, your establishment will need to submit documentation with the application to demonstrate that the situation(s) that gave rise to the suspension has been corrected. The documentation can include, but is not limited to, an adequate corrective and preventive action (CAPA) plan or a corrective action plan (CAP). To verify that the corrections have been implemented, your establishment will be inspected by Health Canada before a licence decision is issued.
Subsequent to the issuance of an MDEL, a notification to Health Canada is required within 15 calendar days should the following changes occur:
Required if you no longer conduct licensable activities under an MDEL.
Health Canada has the authority to cancel your licence if:
If you require changes to information on your MDEL that is not captured under a notification. Examples include changing the activities or classes of medical devices, sites and/or list of manufacturers.
Required if you want to resume selling, importing or manufacturing medical devices following the suspension of your MDEL by Health Canada.
For your MDEL to be reinstated, your establishment will need to submit documentation with the application to demonstrate that the situation(s) that gave rise to the suspension has been corrected. The documentation can include, but is not limited to, an adequate corrective and preventive action (CAPA) plan or a corrective action plan (CAP). To verify the corrections your establishment will be inspected by Health Canada before a licence decision is issued.
Diagram 1. Medical Device Establishment Licence (MDEL) – application screening and review process
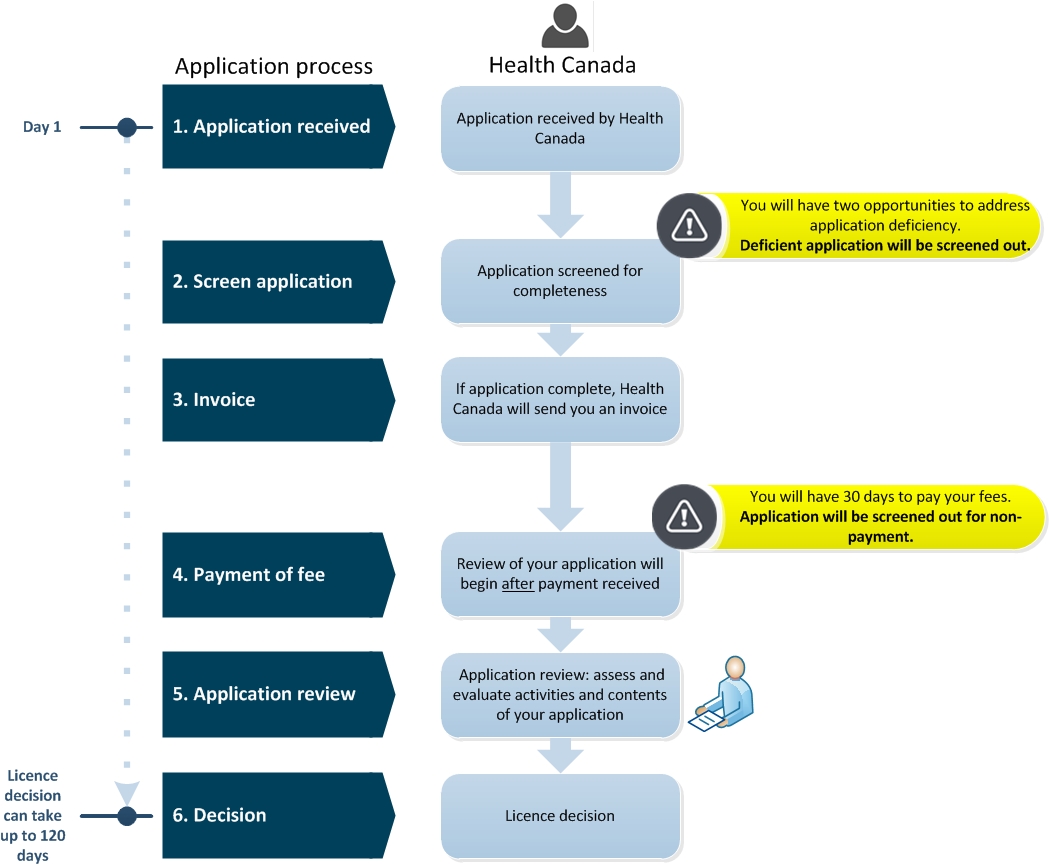
Diagram 1 - Texte description
This flowchart is divided into two sections; the first left section is entitled, "Application Process" and the second right section is entitled, "Health Canada". The left section indicates the steps required in the application process and the right section describes the tasks taken by Health Canada.
Step 1 is entitled "Application received". In this step, Day 1, Health Canada received the MDEL application.
Step 2 is entitled "Screen application". Health Canada screens the application for completeness. Between Step 1 and 2, there is an important point to note, that the applicant will have two opportunities to address application deficiencies. After while, the deficient application will be screened out.
Step 3 is entitled "Invoice". If the application is complete, Health Canada will send you an invoice.
Step 4 is entitled "Payment of fee". Health Canada will begin the review your application after payment is received. There is an important point to note, you will have 30 calendar days to pay your invoice fees. Applications will be screened out for non-payment.
Step 5 is entitled "Application review”. Application review includes the assessment and evaluation activities and content of your application by Health Canada.
Step 6 is entitled "Decision". Health Canada will issue a licence decision. This licence decision can take up to 120 calendar days.
This concludes the flowchart.
If you are submitting a new application, a notification, or a cancellation of an MDEL, you must follow all the applicable requirements under section 45 of the Medical Devices Regulations.
You must complete the MDEL Application Form (FRM-0292) even if you sell or import only one medical device, as quantity does not affect the application.
Include the following in your application:
The manufacturer is the establishment who is listed on the medical device label. It may not be the same establishment who you buy the medical device from. Check your labels before adding the manufacturer to your application.
Distributors:
Manufacturers:
Written procedures will be verified during a regulatory inspection.
If the procedures that were attested to in the MDEL application are not available during an inspection, it will be considered a “false attestation”, which may lead to an MDEL suspension.
Detailed instructions for how to complete the MDEL application form (FRM-0292) are included with the form.
Step 2. Email the completed application form to the MDEL application email account at: hc.mdel.application.leim.sc@canada.ca.
If you submit your application to Health Canada via email, but have other items that need to be sent by mail, include a copy of the email as the cover page to the mailed information. Send to:
Medical Devices Compliance and Establishment Licensing Unit
Regulatory Operations and Enforcement Branch (ROEB)
Jeanne Mance Building – Address Locator 1903C
200 Eglantine Driveway – 3 rd Floor
Ottawa, ON K1A 0K9
The performance standard to issue a decision is 120 calendar days from the day a complete application is received, for the following application types:
After an application is received by Health Canada, it will be screened for completeness. MDEL applications are screened to verify completeness against the following criteria:
If the application is deemed complete, Health Canada will notify you via email that your application has been accepted for further examination/review, and include an invoice for the applicable fees.
Payment is due within 30 calendar days from the date of invoice.
If the fees that are due for an MDEL application are not paid in a timely manner, Health Canada has the authority to withhold services, approvals, rights and/or privileges. Should Health Canada use this authority to stop the review of an application, the period of time where services are withheld does not count toward Health Canada’s 120 day service standard.
Health Canada uses a “clock” to measure performance against the 120 calendar day service standard.
Health Canada will contact you by email during the licensing process if we have questions or need additional information.
A deficiency is when an application cannot be further processed by Health Canada because it does not meet regulatory requirements or the intent/scope of the application is not clear. The applicant is provided an opportunity to submit the missing or incomplete information in order to avoid receiving a negative decision within 30 business days.
In such circumstances, the application clock would be paused for up to 30 business days at a time. If no response is received after the first deficiency notice or if the response that is received is inadequate, a second notice will be issued providing an additional 30 business days to respond.
If no response is received after the second notice is issued to address the same deficiency, or if the received response is inadequate, the application will be rejected.
You may request a status update by emailing hc.mdel.questions.leim.sc@canada.ca, if:
If your application meets all the requirements of section 45 of the Medical Devices Regulations, Health Canada will issue a Medical Device Establishment Licence (MDEL).
Health Canada may refuse to issue you an MDEL if your MDEL application contains false or misleading statement(s).
Health Canada will refuse to issue you an MDEL if the Minister or delegated authority has reasonable grounds to believe that issuing you an MDEL would constitute a risk to the health or safety of patients, users or other persons.
If Health Canada refuses to issue you an MDEL due to these reasons, as listed in section 47 of the Medical Devices Regulations, you will be notified in writing of the reasons for refusal and given an Opportunity to be Heard (OTBH).
Under the Medical Devices Regulations, all active MDEL holders must submit an application for annual licence review (ALR) before April 1 of each year. The purpose of the ALR is to ensure continued compliance with regulatory requirements and to maintain up-to-date information. You must submit this application and pay the fee upon invoicing even if there are no changes to your licence.
Diagram 2. Annual Licence Review timeline

Diagram 2 - Texte description
This flowchart is divided into two sections; the upper section is a monthly timeline and lower section indicates the requirements at each time point noted in the box above.
In December, Health Canada emails Annual Licence Review application package to all active MDEL holders.
In January to February, the timeline that is the recommended submission period of Annual Licence Review application from MDEL holders to Health Canada.
April 1, is the due date for your Annual Licence Review application to Health Canada.
In April to July, the timeline for the processing of ALR applications completed by Health Canada.
This concludes the flowchart.
As a courtesy, Health Canada sends an ALR application package to all MDEL holders at the end of each calendar year. However, it is your responsibility to ensure a complete ALR application is received by Health Canada before April 1 of each year. If you do not receive your ALR package by mid-January, contact Health Canada. See Appendix C – Contact information for a list of contacts.
If you do not submit an application for ALR before April 1 of each year, Health Canada will cancel your MDEL.
You are not permitted to conduct any licensable activities with a cancelled MDEL. See paragraph 51.1(b) of the Medical Devices Regulations for details.
If an MDEL is cancelled and the establishment wishes to resume activities, you are required to apply for a new MDEL and meet the requirements set out in section 45 of the Medical Devices Regulations, as applicable.
After you submit an ALR application:
Under section 48 of the Medical Devices Regulations, licence holders are required to notify Health Canada within 15 calendar days of a change in contact information, as described below.
You must submit a notification to Health Canada within 15 calendar days of:
Notify Health Canada by:
There are no fees associated with making changes, notifications or amendments to your MDEL.
You must inform Health Canada of any change affecting the information on your MDEL (for example, list of manufacturers, change in activity or class of device).
Note: In the case of a change in contact information, you must notify Health Canada within 15 calendar days – see Submitting a notification.
You must inform Health Canada if you choose to cancel your MDEL. Before you submit the application for a cancellation (using FRM-0292), you must ensure that all activities under the MDEL have ceased.
Only the contact person or senior official for the MDEL may submit a cancellation request to Health Canada.
Health Canada may inspect an establishment that had its MDEL cancelled, to verify that all licensable activities have ceased.
Health Canada will review the cancellation request and notify the contact person, via email, that the MDEL has been cancelled and is no longer active.
An MDEL may be suspended or cancelled by Health Canada in accordance with the Medical Devices Regulations. Contact the Medical Devices Compliance and Establishment Licensing Unit (see Appendix C for contact information) should you have any question concerning the suspension or cancellation of your MDEL.
If your MDEL is suspended or cancelled, you must immediately stop importing or selling medical devices. If you fail to stop these activities, Health Canada may take compliance and enforcement actions, as outlined in the Compliance and enforcement policy for health products (POL-0001).
Health Canada’s guidance on Medical Device Compliance and Enforcement (GUI-0073) states that if a regulated party does not voluntarily respond to Health Canada requests, such as inspections, request for copies of procedures, etc., to comply with the Medical Devices Regulations, measures can be considered, including the suspension of an establishment licence.
Health Canada may suspend an MDEL when it has reasonable grounds to believe that:
In making a decision to suspend (under section 49 or 50 of the Medical Devices Regulations), Health Canada will consider the MDEL holder’s compliance history and the risk to the health or safety of patients, users or other persons in allowing the licence to remain valid.
Before suspending your establishment licence under section 49 of the Medical Devices Regulations, Health Canada will send you a written notice explaining:
Alternatively, your establishment licence may be suspended under section 50 of the Medical Devices Regulations, without an opportunity to be heard, to prevent injury to the health or safety of patients, users or other persons. If your licence is suspended under section 50 of the Medical Devices Regulations, Health Canada will notify you in writing and outline:
Health Canada may inspect an establishment that had its MDEL suspended to verify that all licensable activities have ceased.
You may submit a reinstatement application if you want to resume selling, importing or manufacturing medical devices after Health Canada suspends your MDEL.
To reinstate an MDEL after suspension:
To verify the corrections, your establishment will be inspected by Health Canada before a licence decision is issued.
If the MDEL fees have not been paid, Health Canada has the authority to withhold services, approvals, rights and/or privileges.
Once the suspended MDEL has been reinstated, you may resume licensable activities.
The Minister or delegated authority must cancel an MDEL when:
Health Canada may inspect an establishment that had its MDEL cancelled, to verify that all licensable activities have ceased.
Health Canada's authority to reinstate an MDEL is limited to suspended MDELs. If an MDEL is cancelled and the establishment wishes to resume activities, you are required to apply for a new MDEL and meet the requirements set out in section 45 of the Medical Devices Regulations, as applicable.
To apply for an MDEL after cancellation:
Your establishment must submit documentation with the application to demonstrate that the situation that gave rise to the suspension and cancellation has been corrected. The documentation can include, but not limited to, an adequate corrective and preventive action (CAPA) plan or a corrective action plan (CAP).
To verify the corrections, your establishment will be inspected by Health Canada before a licence decision is issued.
If the MDEL fees have not been paid, Health Canada has the authority to withhold services, approvals, rights and/or privileges.
For more information about fees related to MDELs, see the Guidance document – Fees for the Review of Medical Device Establishment Licence Applications and How to Pay Your Establishment Licence Fees.
Once a new MDEL number has been issued, you may resume licensable activities.
To begin a dispute process, or to have the opportunity to be heard about a licence suspension and/or refusal, contact the Medical Devices Compliance and Establishment Licensing Unit (see Appendix C for contact information).
This section outlines some of the key responsibilities of a Medical Device Establishment Licence (MDEL) holder.
Health Canada may inspect anyone who has an MDEL to ensure they comply with the Food and Drugs Act and its Medical Devices Regulations. For more information, see: How Health Canada inspects medical device establishments (GUI-0064).
The manufacturers, importers and distributors of a medical device must each maintain a distribution record for each device. You must also have a documented procedure in place for how you maintain your distribution records, as you attested to in your application for an MDEL.
Your distribution record must contain enough information to allow a complete and rapid withdrawal of any medical device from the market. Your procedures should specify the retention time for your distribution records and how they will be maintained.
Section 55 of the Medical Devices Regulations specifies that:
The manufacturer, importer and distributor shall retain the distribution record maintained in respect of a medical device for the longer of
See sections 52–56 of the Medical Devices Regulations for more information on distribution record requirements, including minimum retention periods for distribution records and how to maintain records to allow for quick retrieval.
Medical device manufacturers, importers and distributors must also maintain records of reported problems for all medical devices they have sold relating to the performance characteristics or safety of the device. These records must include all actions taken to respond to these problems. You must also have documented procedures for complaint handling and recalls. For more information on complaint handling, see the Guidance on Investigation of Reported Medical Device Problems (GUI-0065).
Importers and manufacturers of medical devices are required to provide a preliminary and final report to Health Canada about devices that they sold in Canada for which incidents that took place inside or outside Canada have been brought to their attention.
To avoid duplicate reporting, the manufacturer of the device may allow the importer to prepare and submit the preliminary and final reports on the manufacturer's behalf if the information that must be included is identical. The manufacturer must inform Health Canada, in writing, if such permission has been granted to the importer. However, a manufacturer may not prepare and submit a report on behalf of an importer.
For more information on what types of incidents must be reported and what to include in the report, see the Guidance Document for Mandatory Problem Reporting for Medical Devices.
When recalling a medical device, the manufacturer and importer of that device must each send a report to Health Canada, on or before the recall, outlining the information specified in sections 64–65 of the Medical Devices Regulations.
As soon as possible after completing a recall, the manufacturer and importer must each report to Health Canada the results of the recall and the actions taken to prevent a recurrence of the problem. A manufacturer may allow the importer to submit the information and documents relating to the recall on its behalf by notifying Health Canada in writing if the information that must be submitted is identical for both of them.
ALR: Annual Licence Review Act: Food and Drugs Act DEL: Drug Establishment Licence IVDDs: In Vitro Diagnostic Devices MDEL: Medical Device Establishment Licence MDL: Medical Device Licence Non-IVDDs: Non-In Vitro Diagnostic Devices OTBH: Opportunity to be Heard ROEB: Regulatory Operations and Enforcement Branch
The following definitions explain how terms are used in this guidance document. If there is a conflict with a definition in the Food and Drugs Act, Medical Devices Regulations and/or the Fees in Respect of Drugs and Medical Devices Order, the definition in the legislation prevails.
Custom-made device (as defined in section 1 of the Medical Devices Regulations) - means a medical device, other than a mass-produced medical device, that
Device (as defined in section 2 of the Food and Drugs Act) – Any article, instrument, apparatus or contrivance, including any component, part or accessory thereof, manufactured, sold or represented for use in:
Dispenser (as defined in section 1 of the Medical Devices Regulations) – A person who is a member of a professional governing body and who is entitled, by virtue of their membership in that body, to manufacture or adapt a medical device in accordance with a health care professional’s written directions in order to meet the specific requirements of a patient.
Distributor – A person, other than a manufacturer, an importer or a retailer, who sells a medical device in Canada for the purpose of resale or use, other than for personal use. A person outside of Canada selling medical devices into Canada is also considered to be a distributor.
Health care facility (as defined in section 1 of the Medical Devices Regulations) - A facility that provides diagnostic or therapeutic services to patients. It includes a group of such facilities that report to one common management that has responsibility for the activities carried out in those facilities.
Health care provider – Any person who provides diagnostic or therapeutic services to individuals. This includes emergency first aid services by fire and ambulance departments.
Importer – A person in Canada, other than the manufacturer of a medical device, who is responsible for the medical device being brought into Canada for sale.
Inspection –Monitoring and assessment against the applicable requirements of the Act and its associated regulations. Inspections are routinely conducted based on risk to assess compliance.
Manufacturer (as defined in section 1 of the Medical Devices Regulations) – A person who sells a medical device under their own name, or under a trade-mark, design, trade name or other name or mark owned or controlled by the person, and who is responsible for designing, manufacturing, assembling, processing, labeling, packaging, refurbishing or modifying the device, or for assigning to it a purpose whether those tasks are performed by that person or on their behalf.
Medical device (as defined in section 1 of the Medical Devices Regulations) – A device within the meaning of the Act, but does not include any device that is intended for use in relation to animals.
New applicant – A “new” applicant is a person who has never applied for an MDEL before, including under another name (or previously cancelled MDEL).
Person (as defined in section 2 of the Food and Drugs Act and section 1 of the Medical Devices Regulations) – An individual or an organization as defined in section 2 of the Criminal Code. It includes a partnership and an association.
Procedure – A logically distinct set of activities designed to accomplish a specific task(s). It is concerned with how to achieve the task, rather than what is to be achieved. It defines the work that should be done, and explains how it should be done, who should do it, and under what circumstances. The procedure defines what authority and what responsibility has been allocated, which supplies and materials should be used, and which documents and records must be used to carry out the work.
Record – A document stating results achieved or providing evidence of activities performed.
Retailer – A person who sells a device, or a service using a device, solely to the ultimate consumer.
Many retailers may not be aware whether devices are being purchased by the ultimate consumer for their own use. Where a sale occurs to those who are identifiable as not being the ultimate consumer, the seller is considered to be a distributor, and not a retailer.
Sales agent – A person who is authorized or appointed by a manufacturer to sell or distribute their products as per the attested to procedures, without taking ownership of these products. The sales agent reports to the MDEL holder at one of the sites listed on the MDEL.
Sell (as defined in section 2 of the Food and Drugs Act) – Includes
Senior official – The senior official listed on a Medical Device Establishment Licence (MDEL) application is the person who has direct knowledge of the procedures in place, as confirmed by signing attestations in section 7 on the MDEL application form (FRM-0292).
Site(s) – Any additional building that is used by the MDEL holder (establishment) for keeping the procedures attested to in paragraphs 45(g) to (i) of the Medical Devices Regulations. A P.O. Box is not considered an acceptable site address. A site must be in the same country as the establishment.
Special access (as per Part 2, Medical Devices Regulations) – Access to a medical device for emergency use or if conventional therapies have failed, are unavailable or are unsuitable.
Supplier – Any person, other than the manufacturer, who distributes (sells) a medical device to an MDEL holder for the purpose of import or sale in Canada.
Ultimate consumer / end-user – The individual (also "end-user") who buys or receives a medical device for their own personal use (including within their household) or who receives treatment or is diagnosed with a device from a third party such as a health care facility or provider. Businesses that buy devices (e.g. first aid kits, disposable gloves) solely for use by their employees during work hours are also ultimate consumers, so long as their business does not offer health services to employees or other individuals.
Warehouse – A commercial warehouse would not require an establishment licence if they are only providing storage service and do not purchase, accept products on consignment, or enter into contracts for the sale of medical devices.